Do Gymnosperms Have a Type of Defense Againes Herbivory
Abstract
Glandular trichomes (GTs) are defensive structures that produce and accumulate specialized metabolites and protect plants against herbivores, pathogens, and abiotic stress. GTs have been extensively studied in angiosperms for their roles in defense and biosynthesis of high-value metabolites. In contrast, trichomes of gymnosperms accept been described in fossilized samples, just have not been studied in living plants. Here, we draw the characterization of GTs on young stems of a hybrid white spruce. Metabolite and histological analysis of spruce GTs support a glandular function with aggregating of a diverse array of mono-, sesqui- and diterpenes including diterpene methylesters. Methylated diterpenes take previously been associated with insect resistance in white spruce. Headspeace analysis of spruce GTs showed a profile of volatiles dominated past monoterpenes and a highly diverse array of sesquiterpenes. Spruce GTs appear early during shoot growth, prior to the evolution of a lignified bawl and prior to accumulation of terpenes in needles. Spruce GTs may provide an early, terpene-based chemical defense force system at a developmental stage when young shoots are especially vulnerable to foliage and shoot feeding insects, and before the resin duct system characteristic of conifers has fully adult.
Introduction
Gymnosperms and angiosperms diverged approximately 300 million years ago1. Conifers (Coniferales) correspond a large group of gymnosperms with significant ecological and economic roles in many forest ecosystems and woods-dependent industries. Among others, conifers contribute substantially to global carbon sequestration, soil and h2o conservation, and woods biodiversity. They provide large volumes of valuable woods, fiber and chemic materials for a diversity of traditional forest-products and modern bioproducts industriestwo. Conifers have adapted to a broad range of extreme environments where they take evolved unique chemic and physical defenses to deter the attack of insects and pathogensthree,4. Terpenoid metabolites are a major component of conifer oleoresin defense systems that are produced and stored in specialized secretory structures such every bit resin ducts, resin blisters, and resin cells. These terpenoid accumulating structures are located on the inside of various organs and tissues and are abundantly nowadays in the stem xylem, phloem and cortex. Every bit an internal defense force organisation, they crave tissue damage by, for instance, insect feeding, oviposition or pathogen colonization, to release terpenes as deterrents or toxins. Conifers also emit terpenoids as volatiles from young shoots and needles that tin attract beneficial insects5,vi,7,viii. The emission of terpenes from conifer needles is thought to occur through stomataseven,8,9,10; however, the mechanism of emission and whether other cell-types may contribute remain largely unknown.
Trichomes are typically multicellular structures that beetle from the above-ground surfaces of plants and can be divided into ii master categories: non-glandular (i.due east., hairs) and glandular (i.due east., secretory) trichomes. The most distinct feature of glandular trichomes (GTs) is their chapters to biosynthesize, store and secrete a large diversity of specialized metabolites including terpenoids, alkaloids, polysaccharides, and polyphenolseleven,12,thirteen,xiv. The compounds produced past GTs function in plant defense and protection and also provide an of import source for traditional and modern bioproducts, such as fragrancesxv, food additivesxvi,17, or pharmaceuticals18. Glandular and not-glandular trichomes accept been extensively characterized in a variety of angiosperms, including characterization of their cellular and subcellular structures, transcriptomes, and metabolomes. Despite before reports of uncharacterized "pubescent" structures in gymnosperms and bryophytes19,20, the description of conifer fossils containing trichome-like structures21,22, and the mention of spruce "Drüsenhaare" (i.e. GTs) in some of the classical literature on conifer resins23, to our knowledge, there are no reports on a detailed characterization of trichomes in gymnosperms.
Here we describe the label of GTs on the surface of young shoots of interior white spruce, which represents a naturally occurring genetic admix of white spruce (Picea glauca), Engelmann spruce (Picea engelmannii), and Sitka bandbox (Picea sitchensis). Spruce GTs are formed prior to bud interruption and mature every bit the shoot expands. Histological analysis revealed large intracellular storage compartments and lack of an extracellular secretory cavity. They accrue substantial amounts of mono-, sesqui- and diterpenes, including methylated diterpenes, which have been significantly associated with insect resistance in white spruce (P. glauca)24.
Results
Spruce GTs have a single-cell, globular head with large intracellular storage compartments and begin to develop prior to bud intermission
Nosotros found trichomes densely covering the surface of the stem axis of young, elongating shoots, including the base (pulvini) of needles, of a hybrid white spruce (genotype PG29) (Fig. 1). This genotype has been used equally a reference for spruce genomics25,26 . The bulk of trichomes showed a globular expansion at their noon suggesting a glandular-type trichome (Fig. 1d). Nosotros used scanning (SEM) and transmission (TEM) electron microscopy to investigate these trichomes in more detail. SEM identified two general trichome morphologies, a predominant type with a globular caput (Fig. 1c, cherry-red arrowheads) and less frequent type without globular head (Fig. 1c, white arrowheads). Trichomes with a globular head represented ~ 90% of trichomes on the surface of fully elongated young shoots. In contrast, on younger and all the same elongating stems, trichomes without a globular caput made upward 50% or more than of trichomes (Fig. S1). Elongating stems also had pocket-sized epidermal protuberances which may exist the initial phase of trichome formation (Fig. S1). Trichomes without globular heads may represent an intermediate phase of evolution prior to expansion of globular heads.

Glandular trichomes (GTs) comprehend the young, elongating stems of hybrid white spruce (P. glauca ×P. engelmannii ×P sitchensis, genotype PG29). GTs were imaged with a dissecting microscope (a, b), and by scanning electron microscopy (c, d). GTs embrace the unabridged stem surface, including the pulvini of needles (a). Most trichomes are characterized by a globular head (c, red arrows), but some take a narrow final cell (c, white arrows).
TEM of GT heads showed the head volume filled with dense cytoplasm and several large intra-cellular storage compartments with vacuole-like features and dark electron-dumbo contents after osmium staining (Fig. 2a). TEM images of trichome stalks revealed a multicellular structure also with cytoplasmic-dense cells, each with compartments having vacuole-like features and night electron-dense contents subsequently osmium staining, like to head cells (Fig. 2b). Leucoplasts were institute closely associated with the endoplasmic reticulum in head and stalk cells (Fig. 2c). Head cells also had numerous vesicles in close proximity to the plasma membrane at the periphery of the cell. (Fig. 2d, white arrowheads). These vesicles had a distinct electron density, different from the contents of leucoplasts and vacuole-like compartments, and were absent in stalk cells. GTs had an average height of ~ 180 µm (± 27 s.d., north = 5), with stalk cells having a cross-section of ~ 21 µm (± 3.ane southward.d., north = 6), and the globular head a cross-section at the widest point of ~ 29 µm (± ii.eight s.d., northward = 4).
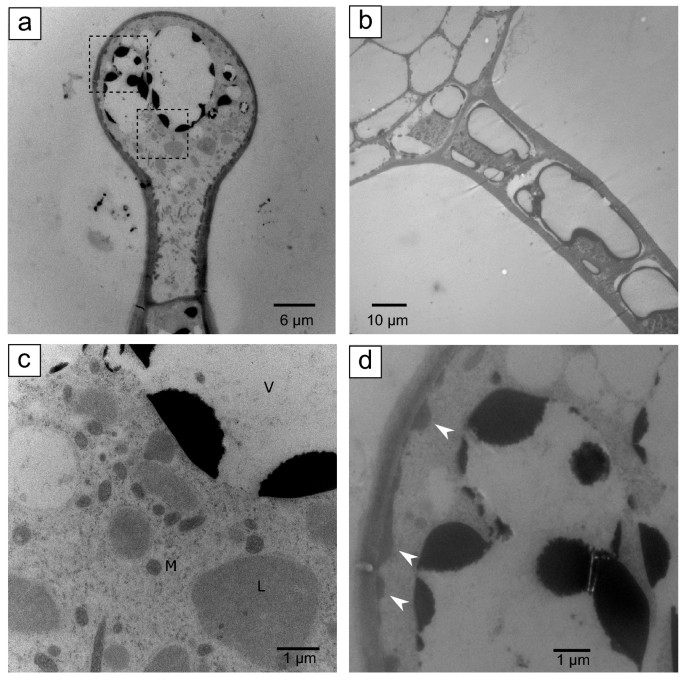
Transmission electron micrographs (TEM) of spruce trichomes. (a) TEM imaging of trichome globular heads showed they consist of a single cell with dense cytoplasm and large vacuole-like compartments with electron dense contents stained with osmium. No bear witness of an extracellular secretory cavity could exist observed. (b) TEM images of trichome stalks showed they are composed of multiple cells with dumbo cytoplasm and vacuole-similar compartments with electron dense contents stained with osmium, similar to globular heads. Higher magnification of areas demarked with dashed lines in (a) are shown in panels (c) and (d). Multiple vesicles with distinct electron density localize side by side to the plasma membrane all around the globular head (d, white arrows). Five, vacuole-like; 50, leucoplast, and M, mitochondria.
To gain a meliorate understanding on the time-grade of GT formation, we collected stems at dissimilar developmental stages from bud swelling to fully elongated shoots and analyzed them past low-cal microscopy (Fig. 3a,b). We found trichomes at all developmental time-points, including buds that showed small protuberances on the surface of the stem axis earlier bud suspension (Fig. 3c,g). At later stages during stalk elongation, the developing trichomes became longer and started developing globular heads (Fig. 3d–f).

Spruce GTs kickoff developing prior to bud suspension. Developing shoots were collected at iv dissimilar developmental stages from the same tree at the same fourth dimension: swollen bud (a, stage I), elongating/flushing stems (a, stages II and Three) and fully elongated stems (b, stage Iv) to rail the formation and appearance of GT. Trichomes could be observed in all four developmental stages (c, d, eastward, f) including in closed, swollen buds (c). Insets in panels (c), (d), and (eastward), show a closer image of developing GT. Toluidine blue staining of stem sections (500 nm thick) later on fixation and embedding in LR white resin, confirmed GT trichomes offset forming at the bud phase (g).
Bandbox GTs accrue mono-, sesqui- and di-terpenes metabolites
To test if spruce GTs contain terpenoid specialized metabolites, which are prominent in other conifer defensive structures, we isolated trichomes, stems (afterwards trichomes had been removed), and needles and extracted their metabolites with methyl tert-butyl ether (MTBE). Gas chromatography-mass spectrometry (GC–MS) of these extracts showed that trichomes contained mono-, sesqui-, and diterpenoids (Figs. 4, 5). The diterpenoids included diterpene methylesters, which are rare in nature. Terpenes present in the trichome extracts included the monoterpenes (−)-α-pinene, (−)-β-pinene, β-myrcene, α-terpinolene, and (−)-bornyl acetate and a sesquiterpene of unknown identity (Fig. 4). Diterpenes in trichomes included methyl-palustrate, methyl-abietate, methyl-neoabietate, sandaracopimaric acid, palustric acid, isopimaric acid, levopimaric acid, and neoabietic acid. Diterpene methylesters occurred naturally in trichomes and were not the issue of trimethylsilyl diazomethane derivatization. The GC–MS traces of trichome extracts revealed three peaks of unknown identity with a m/z 330 and mass spectra with characteristic fragment ions likely originating from diterpene backbones (Fig. 5 and Fig. S2). Extracts of the young stems without trichomes had a like terpene profile compared to GTs, but with a lower ratio of methylated to non-methylated diterpenes and lower relative amounts of the unknown m/z 330 peaks. Needle terpene profiles at this developmental phase were characterized by low relative amounts of monoterpenes and sesquiterpenes except for germacrene-D-4-ol, which had a higher relative amount in needles and was not detected in trichomes or stems (Fig. 4c). Diterpenes were not detected in needle extracts (Fig. 5c).
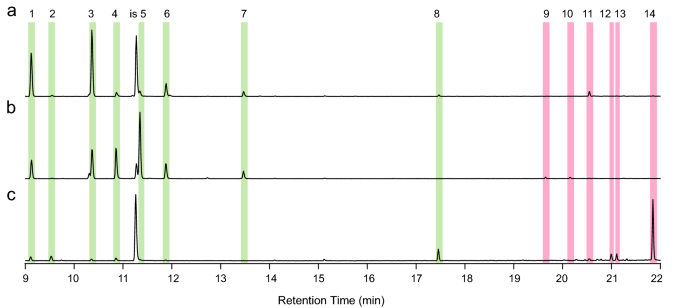
Monoterpenes and sesquiterpenes detected in spruce GT extracts. Shown are total ion chromatograms of metabolite extracts from isolated GT (a), stems subsequently trichomes take been removed (b), and needles (c). The contour of GT was dominated by monoterpenes (green-shaded areas, peaks 1–8) with minor relative amounts of sesquiterpenes (red-shaded areas, peaks 9–14). Stem terpene profiles were also dominated by monoterpenes but with unlike relative amounts compared to GT. Needle terpene profiles were dominated by the sesquiterpene germacrene-D-4-ol and characterized by minor amounts of monoterpenes. Isobutyl benzene was used as internal standard (is). Mono- and sesquiterpenes identified with accurate standards are peak 1: (-)-α-pinene; peak three: (-)-β-pinene; elevation 4: β-myrcene; peak seven: α-terpinolene; peak eight: bornyl acetate; and peak fourteen: germacrene-D-four-ol. Peaks that could not be identified are 2, 5, 6, and nine–thirteen. As well see Tabular array S1 for terpene identification.
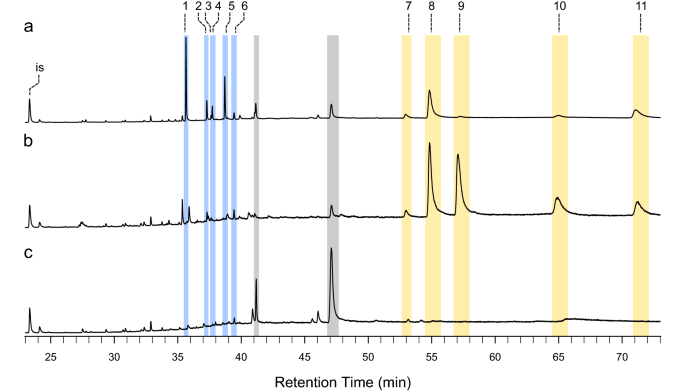
Diterpene resin acids and diterpene methyl esters detected in bandbox GT extracts. Shown are total ion chromatograms of metabolite extracts from isolated GT (a), stems afterward trichomes have been removed (b), and needles (c). GT diterpene profiles contained major peaks of methylated diterpenes (blue shaded areas) including methyl palustrate (summit 1) methyl abietate (tiptop 4), methyl neoabietate (peak five). GT also had several peaks of unknown diterpenes with k/z 330 (peaks 2, 3, and 6). Stem diterpene profiles were characterized by major peaks of diterpenes resin acids sandaracopimaric acid (peak 7), palustric acrid (peak 8), isopimaric acid (peak ix), levopimaric acrid (meridian x), and neoabietic acrid (peak eleven). Needle metabolite extracts only had trace amounts of diterpenes. Eicosene was used as internal standard (is). Methylated diterpenes and diterpene resin acids were identified with authentic standards.
To investigate profiles of volatile terpenes in spruce GTs in more detail, we performed solid-phase microextraction (SPME) sampling coupled with GC–MS with isolated trichomes. SPME sampling of trichome volatiles revealed a highly diverse profile with more 35 different mono- and sesquiterpenes (Fig. 6; Table S1). The major peaks corresponding to volatiles detected by SPME GC–MS represented the aforementioned monoterpenes observed in the MTBE extracts. More than than 16 different monoterpenes were observed in the SPME profiles including camphene, phellandrene, and linalool. SPME GC–MS of sesquiterpenes revealed more than 19 different sesquiterpene compounds.
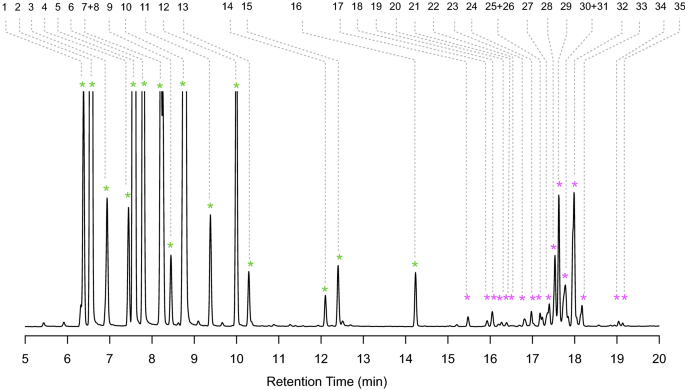
Spruce GTs comprise a big diversity of volatile monoterpenes and sesquiterpenes. Isolated GT were collected in a headspace vial and volatiles were sampled with a solid-phase microextraction (SPME) fiber and analyzed by GC–MS using an HP-v column. GT trichomes volatiles consist by and large of monoterpenes followed by a highly diverse profile of sesquiterpenes. Peaks were identified past a combination of accurate standards, linear retention index calculated with alkane standards, and all-time library hit using the NIST and Whiley libraries as shown in Table S1.
Discussion
Conifers are protected by multiple chemical and physical defenses. At maturity, their massive stems are shielded to the outside with a thick bark formed by layers of lignified and suberized cells. On the within of their stems, the xylem, phloem and bark of mature and young trees contain a reticulate system of resin ducts filled with terpene-rich oleoresinthree,27. In addition, clusters of stone cells with reinforced cell walls28, and layers of polyphenolic cells rich with potentially toxic phenolics29 provide the outer stem tissues with protective barriers. While these defensive structures and their chemicals are typical of fully adult stems, they are missing or are but just emerging in young elongating shoots, including the upmost shoot. The phenology of a delayed development of these major conifer defenses makes conifers vulnerable to herbivores at the time around bud burst, which coincides with the fourth dimension when insects pests such every bit budworms or aphids may be virtually agilethirty,31. The GTs described hither in a hybrid white bandbox, and their specialized terpene metabolites, may provide a developmentally early protection to young emerging shoots. Information technology appears that GTs had previously been disregarded past us and others in the label of conifer defense systemsthree,iv,29. Trichomes are mentioned as taxonomic descriptors for a few bryophytes and gymnosperms including GTs in spruce xix,twenty,32, and they accept been reported in fossilized samples of ancient gymnosperms21,22. Beyond that, we are not aware of a characterization of GTs in conifers, or in any other gymnosperm species. GTs appear to be absent in Sitka spruce (P. sitchensis) and are sparsely found in white spruce (P. glauca) stems (Fig S3), suggesting that GTs in the hybrid white bandbox may originate with the P. engelmannii component in the genetic admix. GT may also exist present in developing stems of sugar pine (Pinus lambertiana), where structures called colleters accept been described to secrete their contents on the surface of young stems33.
Here we showed that spruce GTs accumulate a complex profile of mono-, sesqui-, and diterpenes, including methylated diterpenes and unknown diterpenes. The GT terpene profile was unlike from stems and needles and enriched for methylated diterpenes. Diterpene methylesters accept been reported in a few conifer species including methyl levopimarate and methyl palustrate in rock pine (Pinus pinea), reddish pine (Pinus resinosa) and Scots pino (P. sylvestris)34,35, and the norditerpene o-methylpodocarpic acrid in white spruce (P. glauca)24. Interestingly, higher contents of o-methylpodocarpic acid in white spruce bark were associated with resistance to white pine weevil (Pissodes strobi)24. Diterpene methylesters have non been widely observed in many conifers, which may exist due to the mutual do of diterpene derivatization with methylating reagents for GC–MS analysis, and they may exist more than prevalent than currently thought. To our knowledge, diterpenes with g/z 330 have not been reported in conifers, and may originate from an additional oxidation of diterpene methylesters (Fig. S2). Such oxidation may be catalyzed by cytochromes P450. P450s of the conifer-specific CYP720B subfamily produce diterpene resin acids36,37,38, while other conifer P450s may insert an additional oxygen in the diterpene backbone.
Conifers typically accumulate terpenes extracellularly in the lumen of resin ducts that are embedded inside various tissues throughout the different organs of the tree. To act as a defence force, terpenes must exist released from resin ducts past tissue damage acquired for instance by insect feeding or oviposition or as a event of pathogen colonization. In dissimilarity to resin ducts, spruce GTs lack an extracellular secretory space, but appear to accumulate terpenes intracellularly. Two dissimilar compartments of the GTs may be involved in terpene accumulation: the big vacuole-like compartments nowadays in GT heads and the numerous small vesicles close to the plasma membrane. Volatile terpenes may be released from intact GTs through the plasma membrane or from herbivore damaged GTs. The vesicles next to the plasma membrane of GT caput cells may provide a route for terpene release from intact GTs. Terpenes released from GTs may serve as feeding deterrents or as signals for beneficial insectssix,39. The spruce GTs increase the surface from which terpenes may exist emitted, which in plough may enhance the defensive function of terpene emissions.
Found trichomes have been extensively characterized in angiosperms12,xiv,twoscore. The nowadays label of terpene-producing GTs in spruce provides an opportunity for time to come comparative studies of GTs in angiosperms and gymnosperms, which diverged ~ 300 meg years ago1, with potential implications for a better agreement of the evolution of GTs. Formation of glandular-type trichomes in angiosperms involves various different gene regulatory networks41, suggesting that GTs evolved more than than one time and independently in different species. Studies on fossilized conifer tissues from the Tardily Carboniferous period21,22 propose the possibility that trichomes existed before the radiation issue that gave origin to angiosperms. Future transcriptomic studies on spruce GTs may assist place genes responsible for trichome formation in a gymnosperm, and may as well pb to the genes responsible for diterpene resin acid methylation.
Methods
Plant cloth
Clonally propagated hybrid white spruce trees (P. glauca ×P. engelmannii ×P. sitchensis, genotype PG29, seven years erstwhile) were grown outside in 2-gallon pots. Immature flushing stems (4–half dozen cm long) were collected in the spring. Needles were carefully removed from stems by manus and samples were immediately flash frozen for metabolite analysis, or were cut into 2–3 mm long sections with a razor blade and transferred to fixation buffers for SEM or TEM.
Trichome isolation and metabolite assay
Trichomes were isolated from frozen stems past gentle brushing with a cooled paintbrush over a mortar containing liquid nitrogen and ground with a pestle42,43. To minimize losses of ground trichomes, metabolites were extracted straight in the chilled mortar by adding 2 mL of methyl tert-butyl ether (MTBE) with isobutyl benzene (19 µM), and 1-eicosene (12 µM) as internal standards. Upon thawing, the MTBE-trichome mixture was transferred to a GC vial and extracted at 22 °C with shaking for 2 h. The excerpt was centrifuged at ii,000 ×one thousand for 2 min to remove insoluble materials, and stored at − lxxx °C until GC–MS assay. Stems (subsequently removing trichomes) and needles were ground in a mortar with liquid nitrogen and extracted with MTBE in a GC vial at 22 °C with shaking for 2 h. Diterpenes were analyzed using an Alltech AT-chiliad column (26.half dozen thousand × 0.25 mm ID; 0.25 µm film thickness) with Helium equally carrier gas, prepare at 0.half dozen mL min−1 on an Agilent 7,890/7000A GC/MSD triple Quadrupole organization equipped with an Agilent 7,683 autosampler. The GC oven temperature program was as follows: 50 °C for two min, increment at 5 °C min−1 to 230 °C and hold for 20 min. Injection was done in pulsed splitless mode at 250 °C inlet temperature. Mono- and sesquiterpenes were analyzed on an Agilent HP-5 cavalcade (five% phenyl methyl siloxane, xxx m × 0.25 mm ID; 0.25 µm film thickness) with Helium every bit carrier gas, set at 0.8 mL min-1 on an Agilent 6890A/5,973 GC/MSD system equipped with an Agilent 7,683 autosampler. The GC oven temperature program was as follows: 40 °C for 4 min, increment at vi °C min−1 to ninety °C, followed by increase at 9 °C min−one to 310 °C and hold for 4 min. Injection was done in pulsed splitless mode at 240 °C inlet temperature. Headspace SPME assay was performed using a 100 µm PDMS (polydimethylsiloxane) fiber for extraction of volatiles at xl °C for 10 min and analyzed on an Agilent DB-WAX cavalcade (polyethylene glycol, 59.four m × 0.25 mm ID; 0.25 µm film thickness) with Helium as carrier gas, set at 0.viii mL min-1 on an Agilent 6,890/5,973 GC/MSD system equipped with an Agilent PAL RSI 85 autosampler. The GC oven temperature plan was as follows: 40 °C for 3 min, increase at 10 °C min−1 to 100 °C, followed past increment at three °C min−one to 130 °C, followed by increase at thirty °C min−i to 250 °C and concord for 2 min. Injection was done in split mode with a ratio of 3:1 at 250 °C inlet temperature. Headspace SPME analysis was similarly performed in an HP-5 column with Helium as carrier gas ready at 0.viii mL min−ane on an Agilent 7,890/7000A GC/MSD triple Quadrupole organization. The GC oven temperature program was as follows: 50 °C for ii min, followed by increase at vii °C min−1 to 150 °C, followed past increase at 25 °C min−ane to 300 °C and hold for 2 min. Injection was washed in split mode with a ratio of 3:i at 250 °C inlet temperature. Alkane standards were injected using SPME sampling in the DB-WAX and HP-5 columns to calculate linear retention indexes of mono- and sesquiterpenes. Trichomes used for SPME assay were isolated as described higher up with the exception that they were not ground. Instead, isolated frozen trichomes were transferred with liquid nitrogen direct from the mortar to a head-space vial placed in dry ice. Upon evaporation of the liquid nitrogen, vials were immediately closed and analyzed past SPME GC–MS. Chiral analysis was performed on an Agilent Cyclodex-B column (30 1000 × 0.25 mm ID, 0.25 µm film thickness) with Helium gas as carrier set at 0.9 mL min−1 on an Agilent 6,890/5,973 GC/MSD system. The GC oven temperature programme was as follows: 40 °C for 1 min, increase at 3 °C min−1 to 80 °C, followed by increase at 25 °C min−1 to 240 °C, and hold for 5 min. Injection was done in dissever mode with a ratio of 5:ane at 240 °C inlet temperature. Terpene identification was done by best lucifer to linear retention index reported in the literature; comparison with reference mass spectra from databases of the National Institute of Standards and Technology (NIST) MS library searches (Wiley W9N08L) and relevant literature; and comparing with retention time and mass spectra of authentic analytical standards when bachelor.
Microscopy
Fresh, non-fixed PG29 GT were observed with a WILD M3B dissecting microscope (WILD, Heerbrugg, Switzerland) and imaged with a Nikon D80 digital camera. A series of focus stack images were created manually and processed with Helicon Focus software. Stalk samples for electron microscopy were cut into 2 mm long pieces with a sharp razor blade, and fixed in freshly made 0.one Thousand sodium cacodylate buffer (pH 6.9) containing 1.25% gluteraldehyde and ii% paraformaldehyde. Samples were stock-still under vacuum in a low-temperature microwave (PELCO 3,450 Laboratory Microwave, USA) in 4 cycles of two min each. Samples were removed from fixation buffer, washed with 0.i One thousand sodium cacodylate (pH 6.nine) under vacuum, and postfixed with i% w/v OsO4 in 0.1 Grand sodium cacodylate (pH 6.9). Samples were done in distilled h2o and dehydrated nether vacuum in an ethanol serial until reaching 100% ethanol. Samples used for SEM were transferred into fresh 100% ethanol in ceramic sentry glasses, and the ethanol was allowed to evaporate in a fume hood. Samples were stuck to non-conductive agglutinative sticky tabs mounted on aluminium SEM stubs (Ted Pella, Inc.), coated with 5 nm gold/platinum at two.five kV using a Cressington 208C High Resolution Sputter Coater, and examined with a Hitachi S-2600N Variable Pressure SEM device. The stalk surface of young shoots was examined for three samples obtained from dissimilar trees. Samples for TEM were gently vortexed once in 100% ethanol to detach trichomes from stems. Stem pieces were removed with tweezers and detached-trichomes were infiltrated with Spurr's resin in 10% increment steps of ane h each. Once in 100% Spurr's resin, trichomes were transferred to blocks with fresh resin, centrifuged at i,000g for five min to place them horizontally at the bottom of the block, and polymerized at sixty °C for 16 h. Silver sections 70 nm thick were obtained with glass knives in a Leica UC7 ultramicrotome and mounted in unmarried slot grids. Mounted sections were stained with ii% uranyl acetate for x min, and Reynolds atomic number 82 citrate for 5 min44. Digital images were captured with a Hitachi 7600 TEM (Nissei Sangyo America Ltd.) at 80 kV.
References
-
Bowe, L. M., Glaze, G. & DePamphilis, C. West. Phylogeny of seed plants based on all three genomic compartments: Extant gymnosperms are monophyletic and Gnetales' closest relatives are conifers. Proc. Natl. Acad. Sci. U.s. 97, 4092–4097 (2000).
-
Bohlmann, J. & Keeling, C. I. Terpenoid biomaterials. Found J. 54, 656–669 (2008).
-
Celedon, J. M. & Bohlmann, J. Oleoresin defenses in conifers: Chemic diversity, terpene synthases, and limitations of oleoresin defence under climate change. New Phytol. 224, 1444–1463 (2019).
-
Whitehill, J. Chiliad. A. et al. Functions of stone cells and oleoresin terpenes in the conifer defense syndrome. New Phytol. 221, 1503–1517 (2019).
-
Hilker, G., Kobs, C., Varama, M. & Schrank, Thou. Insect egg deposition induces Pinus sylvestris to attract egg parasitoids. J. Exp. Biol. 205, 455–461 (2002).
-
Mumm, R., Schrank, K., Wegener, R., Schulz, South. & Hilker, Thousand. Chemical analysis of volatiles emitted by Pinus sylvestris later on consecration by insect oviposition. J. Chem. Ecol. 29, 1235–1252 (2003).
-
Martin, D. Thousand., Gershenzon, J. & Bohlmann, J. Induction of volatile terpene biosynthesis and diurnal emission by methyl jasmonate in foliage of Norway spruce. Plant Physiol. 132, 1586–1599 (2003).
-
Miller, B., Madilao, L. 50., Ralph, South. & Bohlmann, J. Insect-induced conifer defense force. White pino weevil and methyl jasmonate induce traumatic resinosis, de novo formed volatile emissions, and accumulation of terpenoid synthase and putative octadecanoid pathway transcripts in Sitka spruce. Institute Physiol. 137, 369–382 (2005).
-
Niinemets, U., Reichstein, Grand., Staudt, Thousand., Seufert, G. & Tenhunen, J. D. Stomatal constraints may affect emission of oxygenated monoterpenoids from the foliage of Pinus pinea. Plant Physiol. 130, 1371–1385 (2002).
-
Harley, P., Eller, A., Guenther, A. & Monson, R. K. Observations and models of emissions of volatile terpenoid compounds from needles of ponderosa pine copse growing in situ: Control by lite, temperature and stomatal conductance. Oecologia 176, 35–55 (2014).
-
Tissier, A. Constitute secretory structures: More than just reaction bags. Curr. Opin. Biotechnol. 49, 73–79 (2018).
-
Schilmiller, A. 50., Terminal, R. L. & Pichersky, E. Harnessing plant trichome biochemistry for the production of useful compounds. Constitute J. 54, 702–711 (2008).
-
Wagner, Yard. J., Wang, Due east. & Shepherd, R. W. New approaches for studying and exploiting an erstwhile protuberance, the plant trichome. Ann. Bot. 93, three–11 (2004).
-
Lange, B. Chiliad. The evolution of plant secretory structures and the emergence of terpenoid chemical diversity. Annu. Rev. Plant Biol. 66, 139–159 (2015).
-
Lange, B. G. et al. Probing essential oil biosynthesis and secretion by functional evaluation of expressed sequence tags from mint glandular trichomes. Proc. Natl. Acad. Sci. USA 97, 2934–2939 (2000).
-
Wang, G. et al. Terpene biosynthesis in glandular trichomes of Hop. Plant Physiol. 148, 1254–1266 (2008).
-
Nagel, J. et al. EST analysis of hop glandular trichomes identifies an o-methyltransferase that catalyzes the biosynthesis of xanthohumol. Constitute Cell 20, 186–200 (2008).
-
Czechowski, T. et al. Artemisia annua mutant dumb in Artemisinin synthesis demonstrates importance of nonenzymatic conversion in terpenoid metabolism. Proc. Natl. Acad. Sci. United states of america. 113, 15150–15155 (2016).
-
Johnson, H. B. Plant pubescence: An ecological perspective. Bot. Rev. 41, 233–258 (1975).
-
Fernald, One thousand. Fifty. Gray's Manual of Botany. (American Book Visitor, 1950).
-
Hernandez-Castillo, G. R., Stockey, R. A., Rothwell, G. W. & Mapes, Chiliad. Reconstructing Emporia lockardii (Voltziales: Emporiaceae) and initial thoughts on paleozoic conifer ecology. Int. J. Institute Sci. 170, 1056–1074 (2009).
-
Rothwell, G. Westward., Mapes, G. & Hernandez-Castillo, One thousand. R. Hanskerpia gen. november. and phylogenetic relationships among the most aboriginal conifers (Voltziales). Taxon 54, 733–750 (2005).
-
Heinrich, M. Das Harz der Nadelhölzer, seine Entstehung, Vertheilung, Bedeutung und Gewinnung (Springer, Berlin, 1894).
-
Tomlin, Eastward. Due south., Antonejevic, Due east., Alfaro, R. I. & Borden, J. H. Changes in volatile terpene and diterpene resin acid composition of resistant and susceptible white spruce leaders exposed to faux white pine weevil harm. Tree Physiol. twenty, 1087–1095 (2000).
-
Birol, I. et al. Assembling the 20 Gb white spruce (Picea glauca) genome from whole-genome shotgun sequencing data. Bioinformatics 29, 1492–1497 (2013).
-
Warren, R. Fifty. et al. Improved white spruce (Picea glauca) genome assemblies and annotation of large gene families of conifer terpenoid and phenolic defence metabolism. Constitute J. 83, 189–212 (2015).
-
Zulak, K. M., Dullat, H. Thousand., Keeling, C. I., Lippert, D. & Bohlmann, J. Immunofluorescence localization of levopimaradiene/abietadiene synthase in methyl jasmonate treated stems of Sitka spruce (Picea sitchensis) shows activation of diterpenoid biosynthesis in cortical and developing traumatic resin ducts. Phytochemistry 71, 1695–1699 (2010).
-
Whitehill, J. Grand. A., Henderson, H., Strong, Westward., Jaquish, B. & Bohlmann, J. Function of Sitka bandbox stone cells as a physical defense against white pino weevil. Institute. Cell Environ. 39, 2545–2556 (2016).
-
Franceschi, 5. R., Krokene, P., Krekling, T. & Christiansen, East. Phloem parenchyma cells are involved in local and distant defense force responses to fungal inoculation or bark-beetle attack in Norway spruce (Pinaceae). Am. J. Bot. 87, 314–326 (2000).
-
Parent, Yard. J., Giguère, I., Mageroy, M., Bohlmann, J. & MacKay, J. J. Evolution of the biosynthesis of two hydroxyacetophenones in plants. Plant Cell Environ. 41, 620–629 (2018).
-
Mageroy, M. H. et al. A conifer UDP-sugar dependent glycosyltransferase contributes to acetophenone metabolism and defense against insects. Plant Physiol. 175, 00611.2017 (2017).
-
Tissier, A. Glandular trichomes: What comes afterwards expressed sequence tags?. Plant J. 70, 51–68 (2012).
-
Sacher, J. A. Structure and seasonal activity of the shoot apices of Pinus lambertiana and Pinus ponderosa. Am. J. Bot. 41, 749–759 (1954).
-
De Simón, B. F., Vallejo, M. C. Yard., Cadahía, East., Miguel, C. A. & Martinez, M. C. Analysis of lipophilic compounds in needles of Pinus pinea Fifty. Ann. For. Sci. 58, 449–454 (2001).
-
Lange, W. & Weissman, G. Untersuchungen der Harzbalsame von Pinus resinosa Ait. und Pinus pinea L. Holz als Roh- und Werkst. 49, 476–480 (1991).
-
Geisler, K., Jensen, N. B., Yuen, M. Yard. Southward., Madilao, L. & Bohlmann, J. Modularity of conifer diterpene resin acrid biosynthesis: P450 enzymes of different CYP720B clades use alternative substrates and converge on the same products. Establish Physiol. 171, 152–164 (2016).
-
Hamberger, B., Ohnishi, T., Hamberger, B., Séguin, A. & Bohlmann, J. Evolution of diterpene metabolism: Sitka bandbox CYP720B4 catalyzes multiple oxidations in resin acid biosynthesis of conifer defense against insects. Plant Physiol. 157, 1677–1695 (2011).
-
Ro, D., Arimura, G., Lau, Southward. Y. W., Piers, Eastward. & Bohlmann, J. Loblolly pino abietadienol/abietadienal oxidase PtAO (CYP720B1) is a multifunctional, multisubstrate cytochrome P450 monooxygenase. Proc. Natl. Acad. Sci. USA 102, 8060–8065 (2005).
-
Hilker, M., Stein, C., Schröder, R., Varama, M. & Mumm, R. Insect egg deposition induces defense responses in Pinus sylvestris: characterisation of the elicitor. J. Exp. Biol. 208, 1849–1854 (2005).
-
Schuurink, R. & Tissier, A. Glandular trichomes: Micro-organs with model status?. New Phytol. https://doi.org/10.1111/nph.16283 (2019).
-
Huchelmann, A., Boutry, M. & Hachez, C. Plant glandular trichomes: Natural cell factories of high biotechnological involvement. Plant Physiol. 175, 00727.2017 (2017).
-
Sallaud, C. et al. Characterization of two genes for the biosynthesis of the labdane diterpene Z-abienol in tobacco (Nicotiana tabacum) glandular trichomes. Institute J. 72, 1–17 (2012).
-
Liu, Y. et al. A geranylfarnesyl diphosphate synthase provides the forerunner for sesterterpenoid (C25) germination in the glandular trichomes of the mint species Leucosceptrum canum. Institute Cell 28, 804–822 (2016).
-
Reynolds, Due east. South. The use of lead citrate at high pH equally an electron-opaque stain in electron microscopy. J. Jail cell Biol. 17, 208–212 (1963).
Acknowledgements
The piece of work was supported with funds to J.B. from the Natural Sciences and Engineering Enquiry Council of Canada (NSERC, Discovery Grant); funds to J.B. from Genome Canada, Genome British Columbia and Genome Quebec in support of the Spruce-Up LSARP Project; and funds to J.M.C and J.B. from the Michael Smith Laboratories Kickstart award program. We thank Dr. Carol Ritland and Ms. Angela Chiang for project and laboratory management. Nosotros admit the fantabulous technical support of the UBC Bioimaging Facility with SEM and TEM.
Author information
Affiliations
Contributions
J.Thou.C designed and performed experiments and analyzed the data. J.M.C and J.B. interpreted the results and wrote the manuscript. J.M.C and J.G.A.West performed SEM and TEM. L.L.M performed GC-MS analysis.
Corresponding writer
Ideals declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open up Access This article is licensed under a Artistic Eatables Attribution 4.0 International License, which permits employ, sharing, adaptation, distribution and reproduction in any medium or format, as long as you lot give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other 3rd party material in this commodity are included in the article'southward Artistic Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Eatables license and your intended employ is non permitted by statutory regulation or exceeds the permitted utilise, you lot will demand to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
Reprints and Permissions
About this article
Cite this article
Celedon, J.M., Whitehill, J.G.A., Madilao, L.50. et al. Gymnosperm glandular trichomes: expanded dimensions of the conifer terpenoid defense arrangement. Sci Rep 10, 12464 (2020). https://doi.org/10.1038/s41598-020-69373-5
-
Received:
-
Accustomed:
-
Published:
-
DOI : https://doi.org/ten.1038/s41598-020-69373-5
Comments
By submitting a comment you concord to bide by our Terms and Community Guidelines. If you notice something calumniating or that does not comply with our terms or guidelines please flag it equally inappropriate.
Source: https://www.nature.com/articles/s41598-020-69373-5
0 Response to "Do Gymnosperms Have a Type of Defense Againes Herbivory"
Post a Comment